您的購物車沒有添加商品!
目錄
1. 氡氣是甚麼?
氡氣(Radon)是一種具放射性,無色,無嗅,無味的惰性氣體,被認為是致癌物質。它是鈾(Uranium)衰變時的一個放射性副產品,存在於大多數的地質結構中。它是92種自己產生的元素之一,並不是人造的污染物。
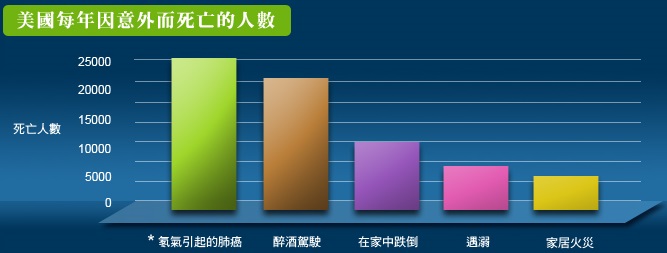
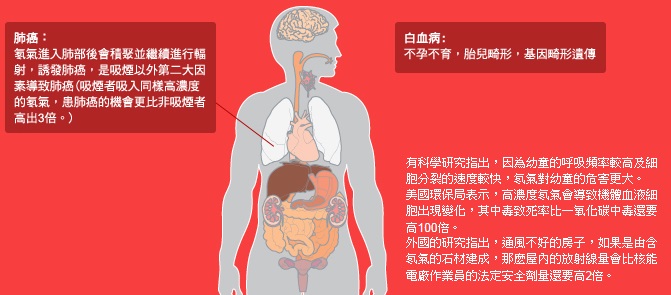
氡氣對人體健康的影響在80年代逐漸受到關注 ── 當氡氣腐朽時它會散發高能量的阿爾法(alpha)微粒,這些微粒會嚴重地損害肺部,引致肺癌。美國環保署估計,1年內約有2萬人因吸入過多氡氣而患肺癌致命,比因醉酒駕駛而意外身亡的人還要多*。
由於氡氣無色無味,只能用特別儀器或設備才能量度它的濃度。 氡氣的量度單位是pCi/l (picocuries per litre of air)。
美國的室外氡氣濃度平均是0.4 pCi/L。美國國家科學院估計,每年約2萬宗因氡氣而患肺癌致命的個案中,有800宗是因為在室外吸入氡氣。而環境中的氡氣濃度越高,患上肺癌的機會就越高──氡氣濃度每上升2.7 pCi/L,患上肺癌的機會就高16% 。
若室內環境通風不足,氡氣會滯留及積聚,濃度肯定比室外高。現時各國並沒有氡氣的安全濃度標準(safety level),因為最安全的情況就是沒有氡氣,但這是沒有可能的,因為氡氣會自然產生。於是,大多數的衛生機構,包括美國環保署,就以4 pCi/l作為氡氣的室內可接受濃度標準(acceptable level)。若室內的氡氣濃度高過4 pCi/l,就需要進行改善工程,以免健康受損。
*氡氣是香港室內空氣質素資料中心(Indoor Air Quality Information Centre)所列的其中一項常見室內空氣污染物。本公司亦另設全面的IAQ測試,歡迎聯絡我們查詢更多詳情。*
物理性質
氡是一種無色無味的氣體,因此,人的感官無法檢測。在標準的溫度和壓力下,一個單原子氣體的氡密度為9.73 kg/m3,大氣層密度為1.217 kg/m3,所以氡的密 度比地球的大氣層大約8倍。氡在室溫中是最重的氣體。
化學性質
氡作為惰性氣體,化學反應不是很活潑。氡的外圍電子數目有八粒,表示已達到八隅體穩定的狀態。 由於氡有放射性,很少出現在化學實驗研究,氡亦難以與其他元素發生反應成為化合物,不過,氡可以由強大的氟化劑如氟發生反應,從而形成氡氟。
同位素
氡的已知同位素有34種,從氡-195到氡-228。其中最穩定的同位素是氡-222是鐳-226的衰變物,會放出α粒子來衰變,半衰期是 3.823日。氡-220是釷的衰變物,又稱「釷射氣」(thoron),會放出α粒子來衰變,半衰期是55.6秒。氡-219是錒的衰變物,又稱「錒射氣」(actinon),會放出α粒子來衰變,半衰期是3.96秒。
2. 氡氣對人體的害處?
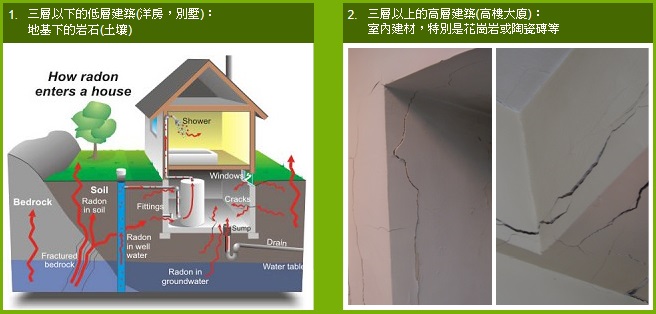
3. 氡氣如何進入室內?
建築材料是室內氡氣的最主要來源,如花崗岩、磚砂、水泥及石膏之類,特別是含放射性元素的天然石材,最容易釋出氡氣。
氡氣從牆壁,地板,或經由地面上的裂縫或空隙進入建築物的地庫,地面層或較高層單位。
對於不同種類的建築物,室內氡氣的主要來源會有所不同。